A Diels-alder Reaction May Best Be Described as
It appears in a lot in organic synthesis problems as it allows to make a cyclic structure from acyclic reactants. Diels Alder Reaction in Organic Synthesis Practice Problems.

Scheme 5 Diels Alder Reactions Of Conjugated Linoleic Acid 16 17 Download Scientific Diagram
The driving force of the reaction is the formation of new σ-bonds which are energetically more stable than the π-bonds.

. Reaction of 9-Anthracenemethanol with N-Ethylmaleimide NEM Reading. The rate of a Diels-Alder reaction is favored by select all that apply electron withdrawing. Diels -Alder Reactions The Diels-Alder cycloaddition of various dienes mostly belonging to 1-vinyl cyclohexenes with o-quinone proceeds very under ultrasound Intramolecular Diels-Alder and Alder Ene Reactions Springer New York 1984.
For more information see ZN p 421-432. How many grams of anthracene would one need in order to perform a Diels-Alder reaction on a 75 mmol scale. C the reaction folows Markovnikovs rule.
Diels-Alder Reaction a very important reaction Reaction between a conjugated diene and an alkene dienophile to give a cyclohexene Diene Dienophile cyclohexene The Diels-Alder reaction is favored by electron withdrawing groups on the dienophile and electron donating groups on the diene. The DielsAlder reaction a concerted 4 2 cycloaddition of a conjugated diene and a dienophile is a powerful tool for the regio- and stereoselective construction of six-membered rings 123456. Diels-Alder reactions are driven solely by adding heat to the reagents.
2 Provide the products for the following Diels-Alder reactions. This reaction produces a 14addition product. Learn vocabulary terms and more with flashcards games and other study tools.
B the products contain rings. D a mixture of endo and exo products are formed. Typically Diels Alder reactions occur at low or moderate temperatures between 25 C and 100 C is a common range.
The Diels-Alder is a stereospecific reaction because the stereochemistry of the dienophile is maintained in the cyclohexene product. More specifically it is classified as a thermally-allowed 2 cycloaddition with. The 42-cycloaddition of a conjugated diene and a dienophile an alkene or alkyne an electrocyclic reaction that involves the 4 π-electrons of the diene and 2 π-electrons of the dienophile.
A trans dienophile yields in the trans product and a cis dienophile yields the cis product. The melting point analysis shows that the product of the Diels-Alder reaction was somewhat impure but the product of the hydrolysis of the Diels-Alder adduct was very pure. The forward reaction is favoured at low temperature whereas the retro reaction is favoured at high temperature.
In organic chemistry the DielsAlder reaction is a chemical reaction between a conjugated diene and a substituted alkene commonly termed the dienophile to form a substituted cyclohexene derivative. Essentially this process involves overlap of the 2p orbitals on carbons 1 and 4 of the diene with 2p orbitals on the two sp 2 -hybridized carbons of the dienophile. Discovered by Otto Paul Hermann Diels and Kurt Alder in 1928.
In the Diels-Alder cycloaddition reaction a conjugated diene reacts with an alkene to form a ring structure. The evidence from the qualitative testing performed in the laboratory confirms the formation of six-membered rings by cycloaddition also known as a Diels-Alder reaction. The DielsAlder reaction is a cycloaddition reaction between a conjugated diene and an alkene.
One of its attractions is the atom efficiency of 100 with no by-products being formed. When these reactants are stimulated by heat they form a cyclohexene product. Start studying Diels-Alder reaction.
Diels-Alder Reaction - McMurry 8th edition Chapter 144-145 UV-vis Spectroscopy McMurry Chapter 147-149 or Mohrig 4th edition Chapter 25 Students work in pairs to perform a Diels-Alder cycloaddition reaction in water solvent. Awarded the Nobel prize in chemistry in 1950. In a Diels-Alder reaction the alkene reacting partner is referred to as the dienophile.
The Diels-Alder reaction is a concerted single step reaction that takes two molecules and creates a six-membered ring. Diels-Alder reactions are concerted stereospecific and follow the endo rule. Before we begin there are a few things to consider when carrying out the reaction.
The Diels-Alder reaction is very important in Organic Chemistry. DielsAlder reaction can be described as a chemical reaction between the substituted alkene ie question_answer Q. In this reaction three double.
Up to 10 cash back This is a standard Diels-Alder reaction. For Diels-Alder we need a cis-diene and an alkene as reactants. Moreover all of the atoms that are participating in the reaction form bonds simultaneously.
O O O O O H O R O OR H O H H H ethylene. The Diels-Alder reaction is a concerted reaction. In comparison retro Diels Alder reactions require more elevated temperatures often above 200 C.
In its original version two new CC bonds are created in the DielsAlder reaction and six-membered carbocyclic systems are obtained. By looking at the reagents and the product we can tell that this is a Diels-Alder reaction. It is the prototypical example of a pericyclic reaction with a concerted mechanism.
E6-1 E19B1 Diels-Alder Reaction in Water. E all bond making and bond breaking occurs simultaneously. The LUMO of the dienophile reacts with the HOMO of the diene in a 42 cycloaddition.
The DielsAlder reaction is favored by the presence of electronwithdrawing groups on the diene and electronreleasing groups on the dienophile. Which of the following best describes a Diels-Alder reaction. A typical example is the reaction of 13butadiene with maleic anhydride.
In fact Otto Diels and Kurt Alder received the Nobel Prize in Chemistry in 1950 for the discovery of this reaction in 1928. A Reaction with High Atom Economy Study Questions 1 Given the following reaction sequence and information draw an energy diagram Energy vs Reaction Coordinate illustrating the major energetic features of the following reaction. The Diels-Alder reaction is a concerted reaction this means it occurs in only one step.
The Diels-Alder reaction is the reaction of a diene with a mono-ene to form a cyclohexene derivative an important reaction for the construction of organic intermediates. A the reaction is highly endothermic. Diels-Alder reactions are normally used to prepare what sized ring.
A B II C III D IV E V HCl CH 6 The Diels-Alder reaction is a concerted reaction this means that A a mixture of endo and exo products is formed B all bond making and bond breaking occurs simultaneously C the products contain rings D the reaction requires heat E the reaction is highly endothermic 7 Which of the following statements is true about a Diels-Alder reaction.

Scheme 4 A Diels Alder Reaction And B Hetero Diels Alder Reaction Download Scientific Diagram

Diels Alder Reactions Of Isoprene 1 And Acrylic Acid 2 Download Scientific Diagram

Diels Alder Reactions Of Citroconic Anhydride And 9 Sub Stituted Download Table

Parent Diels Alder Reaction Bond Lengths In Angstroms A Download Scientific Diagram

A Diels Alder Reaction I E The 4 2 Cycloaddition Using O Mbas Download Scientific Diagram

Diels Alder Reaction Between The In Situ Formed Diene From Nr Cv And Download Scientific Diagram

The Sn2 Reactions R1 And R2 The Diels Alder Reaction R3 And The Download Scientific Diagram

Diels Alder Reaction In A Micellar Solution Of Domim Br Download Scientific Diagram

Diels Alder Reaction Mechanism Stereoselectivity Variations

Scheme 86 Hetero Diels Alder Reaction For The Synthesis Of Fused Download Scientific Diagram

A Dual Role Of Cyclopentadienone In Diels Alder Reactions B Download Scientific Diagram

Radical Cation Diels Alder Reaction With Various Oxidation Methods A Download Scientific Diagram
Asymmetric Synthesis Of Diels Alder Reaction Products Involving Download Scientific Diagram

The Examples Of Uncatalyzed And Catalyzed Quinone Diels Alder Reaction Download Table

Diels Alder Reaction Mechanism Stereoselectivity Variations
Diels Alder Reactions Accelerated By On Water Conditions Breslow 5 Download Scientific Diagram

Scheme 1 Diels Alder Reaction Between 2 3 Dibromo 1 3 Butadiene Dbb Download Scientific Diagram

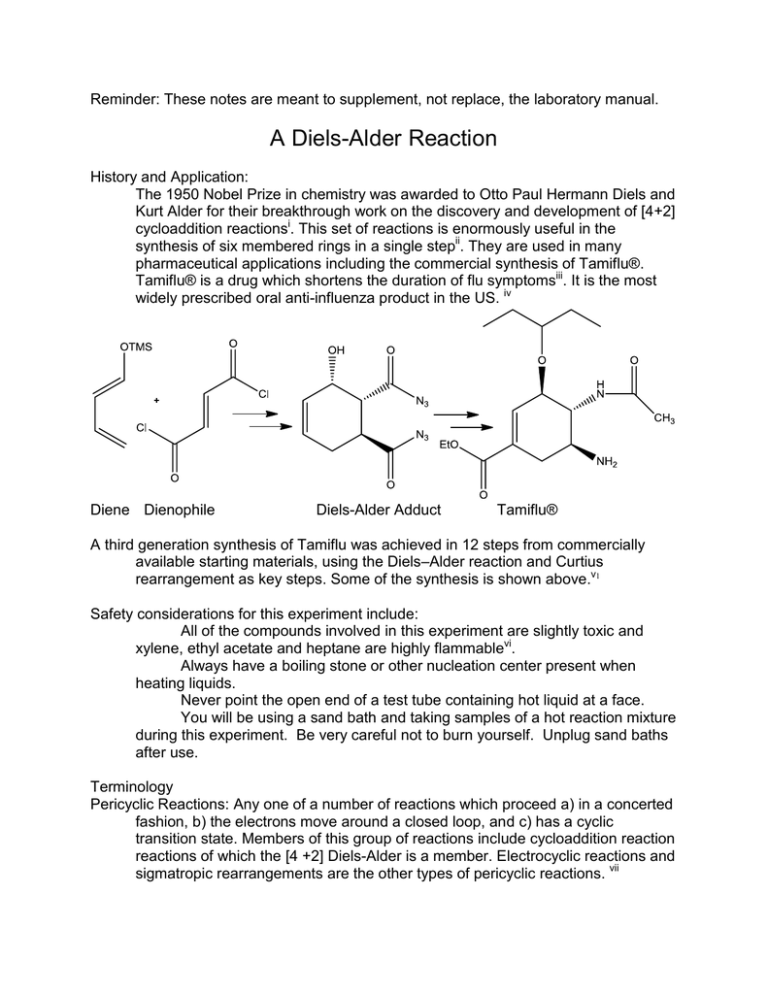
Comments
Post a Comment